Dynamic analysis of muscle activity and biofeedback in any environment

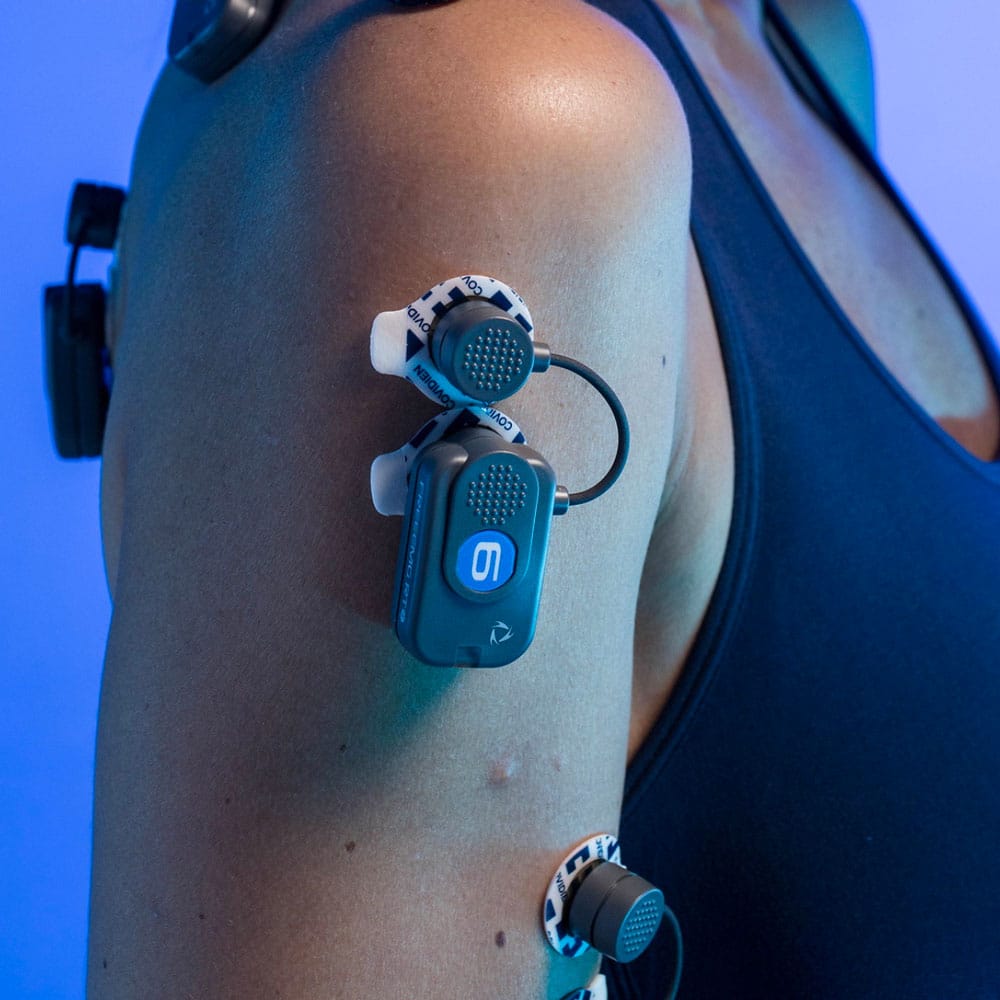
FREEEMG is a wireless device for surface electromyography (EMG) analysis. Signal accuracy, the absence of wires, the lightness and reduced size of the probes are features enabling users to assess any type of movement, for each body part, without altering the natural movement of the person being tested.
Thousands of centres worldwide use our EMG device in various fields such as: research, sports, occupational medicine, gnathology, neurology and orthopaedics.
FREEEMG is a single system used for multiple purposes: from monitoring to diagnostics and up to injury prevention.
The system is provided with a large variety of predefined analysis protocols allow for muscle activity assessment during specific exercises.
The software analyses the collected information and automatically generates graphical results allowing for immediate comparison with normal ranges.
Technical highlights
Battery life
Ultralight probes
EMG Analysis has never been so quick and easy
- Up to 20 probes, selectable among EMG, electrogoniometers and footswitch
- More than 6 hours of non-stop acquisition
- Easy and quick to recharge by clipping onto the proprietary probe charger
- Active and variable geometry electrodes
- Record of miniaturization weighing only 13 grams.
- On-board solid-state buffer memory to secure data in case of any WiFi signal loss
- Up to 20 meters for the data transfer between the probes and the workstation
- Native integration with BTS motion caputre systems, inertial sensors, force platforms, video cameras.
- Integration with third-party systems
- Software for clinical assessment and performance analysis
- Object-oriented software for the development of customised protocols
- Development tools
Wireless Probes
| Surface electrodes | Variable geometry electrodes with snap connectors – 16-bit resolution – acquisition frequency 1KHz |
| Data transmission | Wireless IEEE802.15.4 data transmission (probes – USB receiver) – Real Time |
| Battery | Rechargeable with proprietary charger (snap connector) – lithium-ion polymer |
| Autonomy | Over 6 hours of continuous acquisition |
| Acquisition range | Up to 20 meters (65 feet) in free space (without obstacles) |
| Holter | The solid-state memory allows data storage up to 1 hour and 40 minutes for systems with less than 6 EMG probes, and up to 2 hours for systems with more than 6 EMG probes |
| Memory | On board solid-state buffer memory system |
| Weight | 13 grams (battery included) |
| Size | 41,5×24,8x14mm mother electrode – Ø 16x12mm satellite electrode |
| Certification | Class “IIa” |
USB Receiver
| EMG channels | Up to 10 wireless probes each receiver (no more of 2 receivers allowed) |
| Weight and size | 80 grams – 82x44x22,5mm |
Request information on FREEEMG